Recently, Huang Yanqiang, a researcher at the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and Liu Bin, a professor at Nanyang Technological University in Singapore, have made new progress in the field of carbon dioxide conversion. Related work was published in Advanced Functional Materials.
Carbon dioxide electrochemical reduction reaction is an effective way to achieve recycling of carbon resources. Due to the relative stability of carbon dioxide molecules and the complex products of electrochemical reduction reactions, designing excellent catalysts to reduce overpotentials and improve reaction selectivity and stability is the focus of research on electrochemical reduction of carbon dioxide.
The nitrogen-doped graphene catalyst system is easy to be modeled, easy to transport electrons, and has good stability. It is expected that high-efficiency activation and orientation conversion of CO2 molecules can be achieved through the orientation design of active sites, and the understanding of the CO2 electrocatalytic reaction mechanism and the development of high-performance electrocatalysts. It is of great significance.
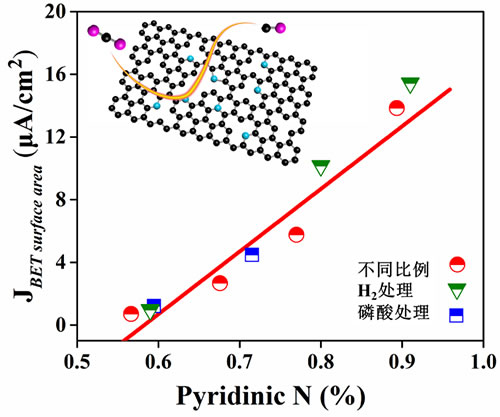
The team prepared a nitrogen-doped graphene catalyst in one step by high-temperature pyrolysis. The catalyst can efficiently and stably convert CO2 to CO. The faradaic efficiency can reach up to 87%, and the catalyst maintains its initial activity after 10 hours of continuous reaction. 98% of activity. In addition, a series of catalysts with different contents of pyridine nitrogen were obtained through precise control of the material. It was found that the pyridine nitrogen content was positively correlated with the activity. Combined with DFT calculation, pyridine nitrogen was proved to be the active center of this catalyst in electrochemical reduction of carbon dioxide. The related research results provide reference and new ideas for the design of high-efficiency CO2 electro-reduction catalysts.
The study was supported by the national key R&D program and the strategic pilot project of the Chinese Academy of Sciences.
Aluminium alloy is a type of metal that is widely used in various industries. This alloy is made by combining aluminium with some other metals, such as copper, magnesium, silicon, and zinc, to enhance its properties. The resulting alloy has improved properties than pure aluminum, such as higher strength, corrosion resistance, and good weldability.
One of the main advantages of aluminium alloy is its lightweight. It is about 1/3 the weight of steel, which makes it an ideal material for transportation applications, such as airplanes, cars, and trains. The alloy's high strength also makes it suitable for structural applications, such as in the construction industry.
The corrosion resistance of aluminium alloy is another benefit. Its protective oxide layer prevents rust and other forms of corrosion, making it an ideal material for outdoor applications, such as building facades, roofing, and window frames. The alloy's good thermal conductivity also makes it suitable for heat exchangers and other heat transfer applications.
Aluminum alloy also has a good aesthetic appeal. It can be easily shaped, welded, and fabricated into various shapes and designs. It can also be anodized or painted to provide a decorative finish.
In conclusion, aluminium alloy is a versatile material with many advantages. Its lightweight, strength, corrosion resistance, thermal conductivity, and aesthetic appeal make it an ideal choice for many applications.
7075 Aluminum,6061 Aluminum,5052 Aluminum,6063 Aluminum
Lizhi Precision Manufacturing Technology Co.,Ltd , https://www.autoindust.com