December 12 news, foreign media, Fast Company, wrote that the digital tablet caused some concerns about the privacy of patients, but supporters believe that this can track the patient's new pills may be just the beginning of edible technology. The following is the main content of the article:

Recently, a "digital pill" has been approved by the US Food and Drug Administration, which may be the beginning of a new wave of edible technology.
The drug is a version of Otsuka Pharmaceutical's Abilify drug (used to treat schizophrenia, bipolar disorder, and depression) that contains electronic sensors that are safe to eat. After swallowing the tablet, it enters the stomach and the sensor sends electrical signals through the body to the bandage-type wearable patch. Next, the patch sends wireless information to the patient's mobile application, recording the amount of medication that has been taken.
"What we did was combine the use of sensors in the patient's body and software analysis in the background." George George, the co-founder and chief medical officer of Proteus Digital Health in Redwood City, California, which provides sensor technology for Otsuka Savage) said.
Their goal is to allow patients to keep track of the doses they take and also allow them to share those records with their doctors—if they like, they can also share it with relatives and caregivers. According to a widely quoted estimate, even in developed countries, only 50% of patients actually take medication according to the doctor's instructions. Therefore, for patients whose condition has not improved, it is difficult for doctors to identify whether they need other medications, or whether they do not take enough doses as required.
“The physician will be able to understand objectively whether their patients have taken the medicines as directed for the first time, which may actually greatly improve the therapeutic effect on patients,†pointed out Robert McQuade, Otsuka's chief strategy officer.
The future of digestible technology
In the future, as more tablets with sensors are approved for use, the beneficiaries may include those who take medication for chronic diseases such as diabetes and hypertension. If they do not take medication on time, they can find that the treatment is not ideal. Proteus also conducted early tests with researchers on the treatment of infectious diseases, including testing tablet technology to help high-risk patients prevent HIV infection, as well as testing expensive drugs for the treatment of hepatitis C.
The patient may even be able to periodically swallow tablets with different types of sensors built into it to automatically track body temperature and acid levels, give them or doctors real-time data, so that there is no need for more invasive treatments or no need to go to the door See a doctor.

Proteus placebo pill with edible sensor
“The pills track specific trajectories in the body and can monitor everything from reactions to the food you eat to your diet and even help with weight loss,†said Assimi, assistant professor at Ohio State University's ElectroScience Lab. Asimina Kiourti said.
At present, the first smart tablet Abilify MyCite has not yet entered the market. Otsuka plans to launch a small-scale launch at the beginning of next year for a limited number of doctors and insurance companies to track patients' responses to sensors and applications.
“The goal is to learn as much as possible about the use in the real world, not the clinical environment,†said Andrew Wright, Otsuka's vice president of digital medicine.
However, the drug has caused some controversy. Critics warn that if this type of technology is popular, patients may be under pressure from doctors, insurance companies, or others to take drugs that they may not want to take.
The psychiatrist and author of “Listening to Prozac,†Peter D. Kramer, wrote in a commentary for Fortune that the drug is “creepy , "Future relatives, judges and even employers may be able to see if patients are on time and dose.
Panic is exaggerated
Andrew Thompson, CEO of Proteus, believes that the panic is exaggerated. He stressed that the company will not cooperate with organizations such as prisons and mental hospitals, because they may force people to wear patches for tracking their medication without seeking their consent.
“We have rejected the opportunity to cooperate with that kind of institution because we think that should not be the destination of these solutions.†He said, “I think that all sorts of speculations about 'Let's imagine all the bad things that could happen' are overestimated. Rendered."
By tracking other types of drug therapy, Proteus has performed satisfactory research on this technology, such as tracking kidney transplant patients taking anti-rejection drugs, tuberculosis patients who are often difficult to adhere to medication, and risk factors (people are more likely to be unable to Hepatitis C patients who can treat expensive drugs that are lethal. A study of eight hypertensive patients found that each patient’s blood pressure dropped after two weeks of regular drug use.
Sometimes, if the patient forgets to take medication, the medical staff can even contact them on the same day and urge them to take the medicine. However, even when the patient takes the drug, the mobile phone is not powered. The patch will report the dose to the mobile phone when the mobile phone is synchronized via Bluetooth, or it will always store the dose record until the next time the patient goes to see a doctor.
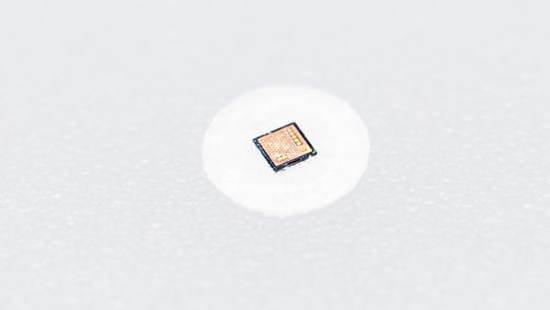
Proteus Digestible Sensors Built into Drug (Not Final Package)
New era, new possibilities
Proteus also collaborates with other pharmaceutical companies outside Otsuka to develop applications, including Novartis. There are also other companies that announce their own smart pill technology trials.
"We really see this as a platform, a platform that can eventually be applied to the entire medical field." Proateus's Savage said, "Of course, the key lies in which applications you have to focus on first, and how to prioritize applications. level."
Thompson noted that the approval to add Proteus's sensors and patches to FDA-approved drugs is much simpler than allowing new drug formulations to be approved. Otsuka applied for approval for Abilify MyCite for the first time in June 2015. The FDA asked it to submit more information last year, and it was not approved until November 13.
Currently, not only researchers from Proteus and its drug company partners are studying digestible technology. An upcoming article in the December issue of the Journal of Anesthesia and Pain describes a similar tablet and patch system from etectRx of Gainesville, Fla., which is designed to track the use of steroids in fracture patients. . The company also announced in September that its technology will be used for research on HIV prevention drugs.
The use of digestible sensors may not only be limited to tracking whether people take medication. "Digestible sensors have been around for some time," says Giotti. "Early digestible sensors were designed to capture photos or videos from the gastrointestinal tract."
Those devices called capsule endoscope cameras can be used for more invasive procedures such as colonoscopy. With the combination of wearable technology and common devices such as smart phones, pill-based sensors will have a greater chance of being used at home rather than at the hospital. Scientists have recently studied how swallowable devices can harness the body's own chemical reactions to generate electricity when passing through the digestive tract.
In Thompson's view, this technology opens up new possibilities for the use of modern digital technologies in the pharmaceutical industry. "Obviously, today the technology may bring a lot of different possibilities." He said, "People should believe that this is a new era of healthcare. The pharmaceutical industry has the opportunity to integrate sensors and software into their toolboxes. "(Lebang)
Partition Door Decorative Film
Smart glass wall has a wide range of application prospects. At night, the window film will block about 95% of the light to give you a high level of privacy.
During daylight hours, any ambient light will reflect the mirror film's surface, making it impossible to see inside. Window film can help stop people from seeing in at a fraction of the cost of new windows. Decorative Films, LLC provides Decorative Window Film, stained glass window film, window privacy film, and frosted glass films.
Window Privacy Film Rainbow Clings 3D Decorative Window Vinyl Stained Glass Window Decals Static Cling Window Sticker Non-Adhesive. Long story short, window films will not harm your plants. Window films filter out many parts of the sun's rays, but not to an extent which harms plants.
This is because higher quality window films-the types we use here at AP Corp-don't filter out the important part of the sun's rays: visible light.
Please contact us now! We can give you the best price. In general, the application of smart dimming glass mainly depends on the specific situation, and it does not need to be selected for decoration.
Partition Door PDLC Film,Projection Partition Glass,Privacy Partition Door,Switchable Partition Door,Electronic Glass Door Film
Tianqixun Technology , https://www.tqxdisplay.com