Abstract 1. A biomimetic iron catalyst for the reduction of nitrates and perchlorates (Abioinspiredironcatalystfornitrateandperchloratereduction)
1. A biomimetic iron catalyst for reducing nitrate and perchlorate
(Abioinspired iron catalyst for nitrate and perchlorate reduction)
Nitrate and perchlorate are widely used in industrial technology, synthetic materials and agriculture, but they have become common water pollutants. Industrial processes utilizing chemical reduction of these oxyanions typically require harsh conditions, while microorganisms can efficiently effect enzymatic reduction. Inspired by the activity of nitrate reductase and (high) chlorate reductase, Courtney L. Ford et al. developed an iron catalyst. The catalyst has an auxiliary ligand that assists in the deoxygenation of the oxyanion. When the oxyanion is reduced, it forms iron (III)-oxygen, and in the presence of protons and electrons, the catalyst is regenerated and water is released. (Science DOI: 10.1126/science.aah6886)
2. The weakening of ammonia, water and xenon XH bonds caused by coordination
(Coordination-induced weakening of ammonia, water, and hydrazine X–H bonds in a molybdenum complex)
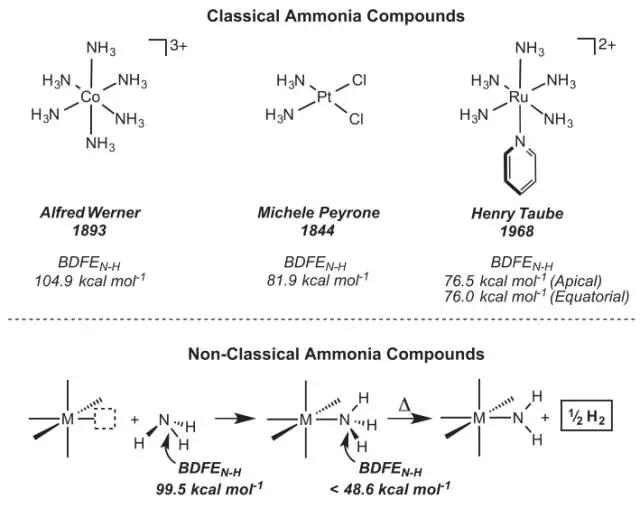
Although many transition metal complexes that bind ammonia or water ligands have been characterized in the past century, little is known about how coordination affects the strength of nitrogen-hydrogen and oxygen-hydrogen bonds. MátéJ. Bezdek et al. introduced the synthesis of a triple acridine ligand and a phosphine ligand-supported molybdenum ammonia complex, which reduces the dissociation free energy of nitrogen-hydrogen bonds from 99.5 (gas phase) to 45.8 kcal. /mol (experimental measurement), very close to the 45 kcal/mole calculated by density functional theory. This weakening of the bond contributes to the spontaneous release of dihydrogen under mild heating and the hydrogenation of styrene. A similar molybdenum complex promotes the release of dihydrogen in the coordinated water and hydrazine. Electrochemical experiments and theoretical calculations illustrate the contribution of metal redox potential and acidity to this effect. (Science DOI: 10.1126/science.aag0246)
3. Gradient band gap perovskite solar cell
(Gradedbandgap perovskite solar cells)

Organic-inorganic halide perovskite materials have become a very attractive alternative to conventional solar cell materials. Their high light absorption coefficient and long diffusion length mean high power conversion efficiency, and the design of perovskite-based single-gap and tandem solar cells has indeed produced impressive performance. One way to further enhance the use of solar spectroscopy is the gradient bandgap, but this has not been achieved in perovskite materials. Onur Ergen et al. demonstrated a gradient energy gap perovskite solar cell with an average steady-state conversion efficiency of 18.4% and a maximum of 21.7% under a non-reflective coating. They analyzed the experimental data to obtain a high fill factor of about 75% and a high short circuit current density of up to 42.1 mA cm-2. The cell is based on the structure of two perovskite layers (CH3NH3SnI3 and CH3NH3PbI3−xBrx) combined with GaN, single-layer hexagonal boron nitride and graphene aerogel. (Nature Materials DOI: 10.1038/NMAT4795)
4. Localized dielectric breakdown and anti-reflective coating in metal oxide semiconductor photoelectrodes
(Localized dielectricbreakdown and antireflection coating in metal–oxide–semiconductorphotoelectrodes)
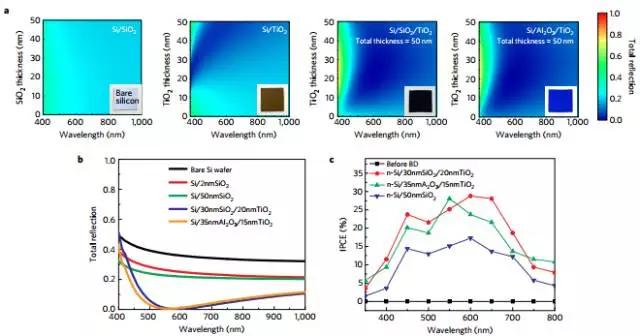
The use of silicon-based photoelectrodes for solar fuel production over the past decade has generated great interest, but the main challenge is the vulnerability of silicon to corrosion. An insulating film is used as a protective layer in the metal-insulator-semiconductor structure. Although it can prevent corrosion, it must also allow transmission of low-impedance carriers, so a trade-off between stability and high efficiency is usually required. Ji et al. proposed and demonstrated a simple method of separating the insulator from corrosion and hindering carrier transport by local dielectric breakdown. This approach allows the insulator to be thicker, thereby enhancing stability while also achieving low impedance carrier transport required for high efficiency. Moreover, this method can be applied to various oxides such as SiO2 and Al2O3. In addition, this method is equally applicable to silicon, III-V compounds and other light absorbing materials used for photocathodes and anodes. The thick metal oxide layer can also be used as an anti-reflective coating film for improving light absorption efficiency. (Nature Materials DOI: 10.1038/NMAT4801)
5. Material design and manufacturing of next-generation flow battery technology
(Material design and engineering of next-generation flow-battery technologies)

The spatial separation of electrolytes and electrodes is a major feature of flow battery technology, which allows them to be independent of the total energy content and energy/power ratio. The concept of flowing electrolytes not only provides a cost-effective approach to large-scale energy storage, but has also recently been used to develop a wide range of new hybrid energy storage and conversion systems. The emergence of flow-type lithium ions, organic redox active materials, metal-air batteries and photoelectrochemical cells has brought new opportunities for advanced energy storage technology. Minjoon Park et al. introduced and reviewed recent advances in conventional aqueous redox flow batteries and next-generation flow batteries, highlighting the latest innovative alternatives and outlining their technical feasibility in long-term and large-scale electrical energy storage devices. Sex, and the limitations that need to be overcome. (Nature Reviews Materials DOI: 10.1038/natrevmats.2016.80)
6. Self-assembly of nanoparticles into bionic shell-like nanoshells
(Self-assembly of nanoparticlesinto biomimetic capsid-like nanoshells)
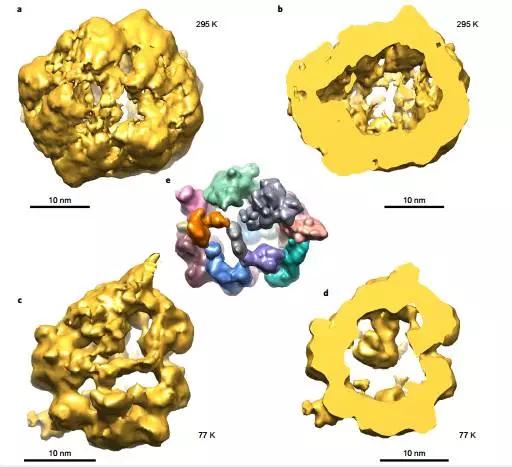
Nanoscale compartments are one of the basic elements of a living system. Capsids, carboxylases, exosomes, vacuoles, and other nanoshells can be easily formed by self-assembly of biomolecules (eg, lipids or proteins) rather than from inorganic nanomaterials because inorganic nanomaterials are difficult to replicate. surface. Yang et al. showed that the non-stabilizer polydisperse inorganic nanoparticles can spontaneously organize into porous nanoshells. The combination of water-soluble CdS nanoparticles and self-constrained spherical capsules is the result of proportional adjustment of electrostatic force, dispersion force and other colloidal forces. These cannot be accurately described by the Derjaguin-Landau-Vervey-Overbeek theory, which reveals the appearance of nanoshells and some of their stabilizing mechanisms. The assembled morphology under different conditions almost exactly matches the transmission electron microscope tomography data. This study bridges the gap between biological and inorganic self-assembled nanosystems and provides a new approach to the spontaneous compartmentalization of a wide range of inorganic nanoparticles. (Nature Chemistry DOI: 10.1038/NCHEM.2641)
7. Tracking ultra-fast motion of individual molecules by femtosecond orbit imaging
(Tracking the ultrafastmotion of a single molecule by femtosecond orbital imaging)
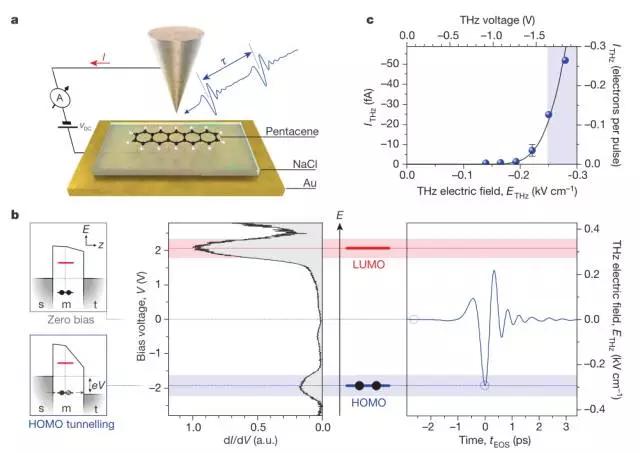
Observing the movement of a single molecule on its intrinsic time scale is known to be one of the important goals of modern nanoscience and requires ultra-fast transient resolution measurements. Steady-state experiments can achieve the necessary spatial scales, either by direct imaging of a single molecular orbital using a scanning tunneling microscope, or by using a tip-enhanced Raman and luminescence spectrum with sub-molecular resolution. But directly tracking the intrinsic kinetics of a single molecule faces a huge challenge, that is, the interaction with the molecule must be limited to the femtosecond time window. For individual nanoparticles, this ultrafast time limit has been demonstrated by combining a scanning tunneling microscope with so-called lightwave electronics. Cocker et al. rely on ultrafast terahertz scanning tunneling microscopy to detect tunneling regions of selected states in which the peaks of the terahertz electric field waveform instantaneously open a tunneling channel through a single molecular state. It is thus possible to remove a single electron from the highest occupied molecular orbital of a single pentacene molecule within a time window that is shorter than one oscillation period of the terahertz wave. Cocker et al. used this effect to record a snapshot image of the orbital structure of approximately 100 femtoseconds at sub-angstrom resolution and reveal the coherent molecular vibration directly in the terahertz frequency time domain by pump/probe measurements. It is expected that if this atomic resolution method is combined with lightwave electronics, it will open the door to visual ultrafast photochemistry and single-orbit-scale molecular electronics. (Nature DOI: 10.1038/nature19816)
8. Multifunctional two-phase hydrolysis catalyst
(Amultifunctional biphasic water splitting catalyst tailored for integration with high-performance semiconductor photoanodes)

Yang et al. used a plasma-enhanced atomic layer deposition to prepare a multifunctional nanocatalyst with high performance photoelectrochemical energy conversion. The controlled synthesis of Co3O4/Co(OH)2 film can simultaneously provide high activity catalytic performance for hydrolysis, which is beneficial to effective interface charge transport and improve the long-term stability of the active interface. These films are composed of dense, continuous spinel structure Co3O4 nanocrystals. Co3O4 nanocrystals are not susceptible to phase transition and can block ion permeation, thus providing effective protection for the substrate. Moreover, the structurally disordered and chemically unstable second phase Co(OH)2 ensures a high concentration of catalytically active sites. The application of this film to photovoltaic p+n-Si brings good performance to crystalline silicon photoanodes. (Nature Materials DOI: 10.1038/NMAT4794)
This article is authorized by the new material online (micro signal: xincailiaozaixian), if other media need to reprint, please contact the new material online small series (micro signal) Citric Acid is white or almost white, crystalline powder, colourless crystals or granulars, odoless has a strongly ACID taste, very solubleinwater, freely soluble in ethanol. It`s mainly used as acidulant,flavoring agent, preservative and antistaling agent in food and beverage industry.
It is also used as antioxidant,Plasticizer and Detergent in chemical,cosmetics and cleaning industries.
Citric Acid
Citric Acid 99.5%,Industrial Grade Citric Acid,Citric Acid Anhydrous,Citric Acid Monohydrate,Food grade Citric Acid
Yucheng Jinhe Industrial Co.,Ltd , https://www.jinhetec.com







