Abstract Recently, researchers from Rice University and Moscow have made a groundbreaking discovery published in the *Nano Letters* journal by the American Chemical Society. They used a pressureless method to develop ultra-thin diamond films, opening up new possibilities in materials science. The team found that under specific conditions, chemical-induced phase transitions can lead to the complete growth of diamond films without the need for high-pressure equipment or complex setups.
This innovative diamond film, known as "dimane," exhibits all the remarkable properties of traditional diamond, including exceptional thermal conductivity, hardness, and semiconductor characteristics. Its ultra-thin nature makes it ideal for use in advanced nanoelectronics and micro-scale devices.
Sorokin, a senior researcher at the Institute of Superhard Materials and New Carbon Materials in Moscow, highlighted the potential applications of dimane. He explained that in nanocapacitors, this material could serve as an ultra-thin dielectric layer, while in nanoelectronic components, it could act as a highly efficient insulator. Additionally, its unique properties make it a promising candidate for use in nanodiodes and other next-generation electronic systems.
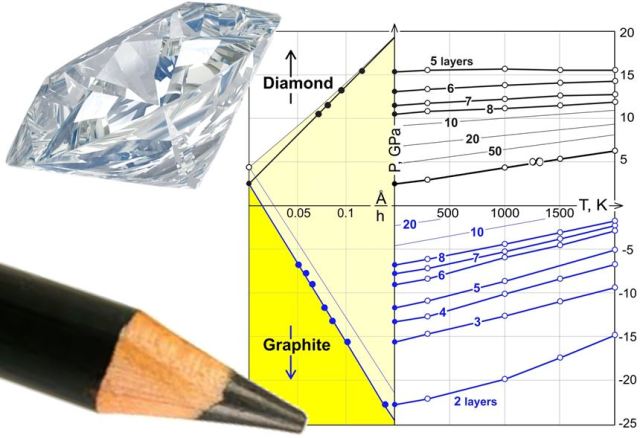
The image shows a phase diagram of a chemically fabricated diamond film developed by scientists at Rice University and Moscow. Diamane is derived from a single-layer graphene.
The phase diagram illustrates how the graphene layer transforms into a flawless diamond crystal, influenced by temperature, pressure, and other factors. This process represents a complete transition from a two-dimensional material to a three-dimensional diamond structure.
Unlike traditional methods, which often require high temperatures and pressures, this new technique uses chemical reactions to induce the transformation. Scientists like Richard Feynman had previously explored similar concepts, using surface hydrogenation to create graphene. Now, the same approach has been applied to produce diamond films through a controlled chemical process.
Through computer modeling, researchers simulated the atomic interactions during the growth of dimane, including the role of hydrogen atoms in catalyzing the reaction. Their findings revealed that when experimental conditions are met, graphene can be converted into diamond with minimal energy input.
According to Yakobson, the phase diagram for dimane is unique because the final structure depends on the thickness and number of graphene layers. These parameters introduce new variables that were previously unexplored in diamond synthesis.
In their experiments, the team used hydrogen as a catalyst. When hydrogen interacts with carbon atoms in graphene, it removes an electron, breaking a bond and leaving an electron on one side. This creates a favorable environment for bonding, requiring almost no external pressure.
If multiple layers of graphene are used, the reaction can trigger a domino effect. Hydrogen moves from the top layer down, gradually converting each layer into diamond. Once fully reacted, the resulting structure becomes a perfect diamond film.
While chemical vapor deposition (CVD) is another method for producing diamond films, it often results in polycrystalline structures with inherent defects. In contrast, dimane offers a more uniform and defect-free alternative, making it suitable for high-performance applications such as wide-bandgap semiconductors and advanced electronic devices.
This breakthrough demonstrates the power of chemistry in shaping the future of materials science and opens the door to new innovations in nanotechnology and beyond.Calender Reducer,Calender Gearbox Reducer,Plastic Calender Machine Gearbox,Calender Gear Box Reducer
JIANGYIN TIANCHENG MECHANICAL EQUIPMENT CO., LTD. , https://www.gearboxjc.com